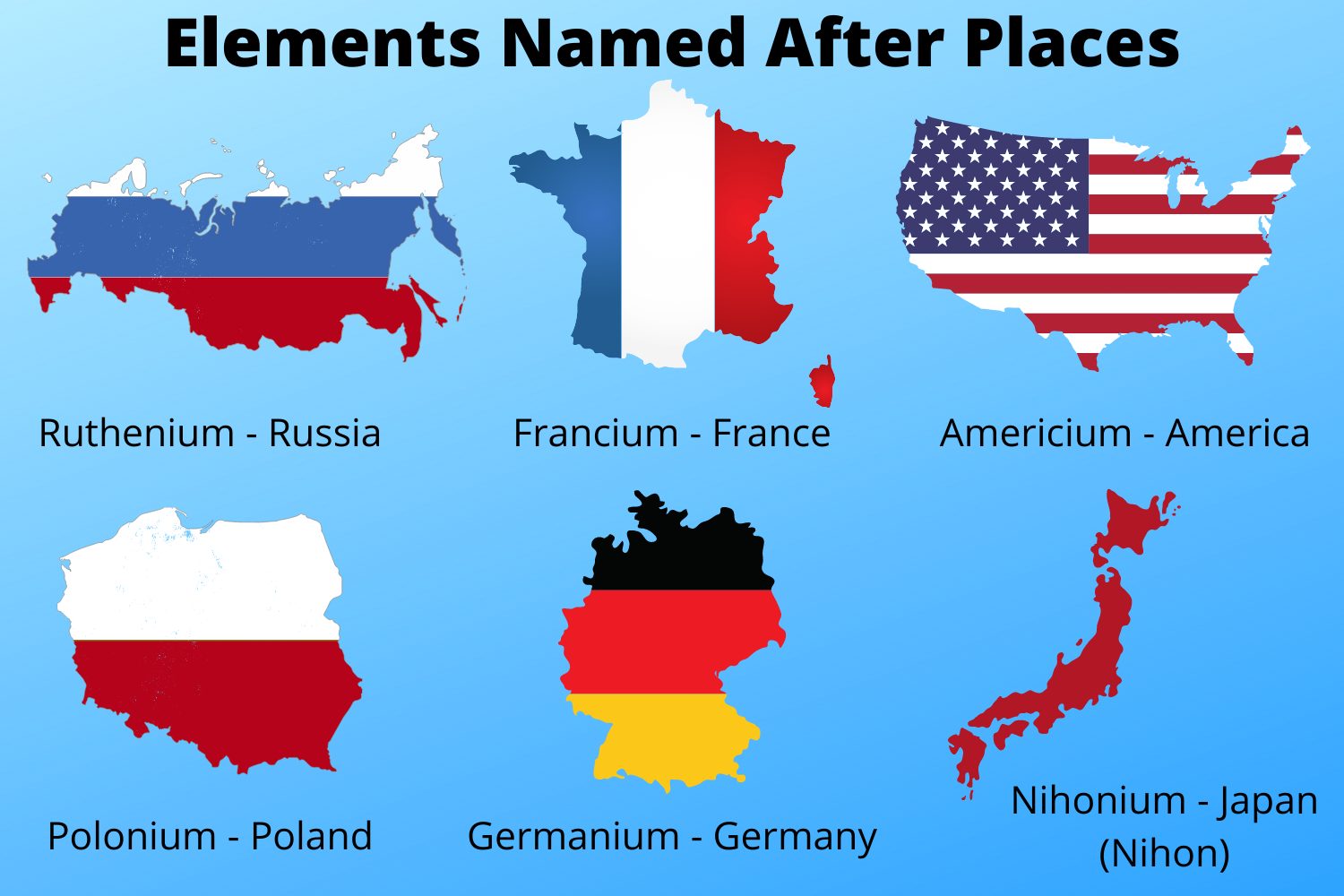
Americium is a radioactive element named after the Americas. It was discovered in 1944 by a team of scientists at the University of California, Berkeley. Americium is a member of the actinide series, and its atomic number is 95. It is a relatively soft metal with a silvery-white appearance. Americium is used in a variety of applications, including smoke detectors, neutron sources, and medical imaging.
Americium is an important element for a number of reasons. First, it is a relatively long-lived radioactive element, with a half-life of 432 years. This makes it useful for applications where a long-lived source of radiation is needed. Second, americium is a relatively easy element to produce. It can be produced by bombarding uranium with neutrons in a nuclear reactor. Third, americium is a versatile element that can be used in a variety of applications. It is used in smoke detectors to detect the presence of smoke particles. It is used in neutron sources to produce neutrons for a variety of purposes, including medical imaging and cancer treatment. Americium is also used in medical imaging to produce images of the inside of the body.
Americium is a fascinating element with a wide range of applications. It is an important element for both scientific research and practical applications.
What Element is Named After a Continent?
Americium, a radioactive element named after the Americas, holds great significance in various scientific and practical fields.
- Discovery: University of California, Berkeley, 1944
- Atomic Number: 95
- Symbol: Am
- Properties: Radioactive, silvery-white metal
- Applications: Smoke detectors, neutron sources, medical imaging
- Importance: Long-lived radioactive element, easy to produce, versatile
- Benefits: Enhanced smoke detection, cancer treatment, medical imaging advancements
- Historical Context: Named after the discovery of the Americas
- Environmental Impact: Radioactive waste management considerations
- Future Prospects: Ongoing research for new applications in energy and medicine
Americium's unique properties and wide-ranging applications make it an essential element in modern society. Its discovery has paved the way for advancements in diverse fields, from safety to healthcare. Understanding the various aspects of americium deepens our appreciation for the element's significance and its potential to shape future technologies.
Discovery
The discovery of americium at the University of California, Berkeley in 1944 marked a significant milestone in the history of element discovery. A team of scientists led by Glenn T. Seaborg and Ralph A. James bombarded plutonium with neutrons in a cyclotron, successfully synthesizing americium. This groundbreaking achievement expanded the known elements beyond uranium and opened up new avenues for scientific research.
The discovery of americium is intricately connected to the element's name, which pays homage to the Americas. This naming convention reflects the scientific community's recognition of the element's discovery on the American continent. The choice of "americium" as the element's name symbolizes the significant contributions of American scientists to the field of nuclear chemistry.
Understanding the connection between the discovery of americium at the University of California, Berkeley in 1944 and its naming after a continent provides valuable insights into the history of scientific discovery and the international collaboration that drives scientific progress. This understanding underscores the importance of recognizing and celebrating the achievements of scientists and institutions that have shaped our knowledge of the elements and the world around us.
Atomic Number
The atomic number of an element is the number of protons in its nucleus. Americium, the element named after the Americas, has an atomic number of 95. This means that every atom of americium has 95 protons in its nucleus. The atomic number is a fundamental property of an element and determines its chemical properties.
The atomic number of americium is significant because it places the element in the actinide series of the periodic table. The actinides are a group of 14 elements that are all radioactive. Americium is the fourth actinide element, after actinium, thorium, and protactinium.
The atomic number of americium also determines its chemical properties. Americium is a metal that is highly reactive. It tarnishes in air and reacts with water to form americium hydroxide. Americium is also a strong reducing agent and can easily lose electrons.
The understanding of the atomic number of americium is essential for a number of reasons. First, it allows scientists to classify americium correctly in the periodic table. Second, it helps scientists to understand the chemical properties of americium and how it will react with other elements. Third, it allows scientists to develop new applications for americium.
Symbol
The chemical symbol for americium is Am. This symbol is derived from the element's name, which in turn is derived from the Americas. The use of the symbol "Am" is essential for a number of reasons.
First, the symbol provides a shorthand way to refer to the element. This is important in scientific writing and communication, where it is often necessary to refer to elements by their symbols rather than their full names.
Second, the symbol helps to distinguish americium from other elements. This is important because americium is a radioactive element and it is important to be able to identify it quickly and easily.
Third, the symbol provides information about the element's atomic number. The atomic number is the number of protons in the nucleus of an atom of an element. Americium has an atomic number of 95, which means that every atom of americium has 95 protons in its nucleus. This information is important for understanding the chemical properties of americium.
The symbol "Am" is an important part of the element americium. It provides a shorthand way to refer to the element, helps to distinguish it from other elements, and provides information about its atomic number.
Properties
Americium, the element named after the Americas, possesses distinct properties that set it apart from other elements. Its radioactivity and silvery-white appearance play significant roles in its applications and necessitate careful handling.
- Radioactivity: A Unique Attribute
Americium's radioactivity stems from its unstable atomic structure. This property makes it a valuable source of radiation for various applications, such as smoke detectors, neutron sources, and medical imaging. However, proper handling and storage are crucial due to the potential health hazards associated with radiation exposure.
- Silvery-white Appearance: A Visual Characteristic
Americium's silvery-white appearance is a result of its metallic nature. This property contributes to its usefulness in certain applications, such as electrodes and alloys. However, it also requires specific storage conditions to prevent tarnishing and maintain its physical integrity.
The combination of americium's radioactivity and silvery-white appearance makes it a unique element with specialized applications. Understanding these properties is essential for handling, storing, and utilizing americium safely and effectively.
Applications
Americium, the element named after the Americas, finds diverse applications in various fields, including smoke detection, neutron sources, and medical imaging. These applications leverage americium's unique properties, showcasing its versatility and importance.
- Smoke Detectors: Enhancing Safety
Americium's radioactivity is harnessed in smoke detectors to ionize air molecules. When smoke particles enter the detector, they disrupt the ionization process, triggering an alarm. This life-saving application of americium contributes to fire prevention and safety.
- Neutron Sources: Facilitating Research and Medicine
Americium-241 is a key component in neutron sources used for scientific research and medical applications. These sources emit neutrons, which are essential for studying atomic structures, analyzing materials, and treating certain types of cancer.
- Medical Imaging: Advancing Diagnostics
Americium-241 is also utilized in medical imaging techniques such as bone mineral densitometry. It emits gamma rays that interact with bones, providing valuable insights into bone health and osteoporosis detection.
The applications of americium in smoke detectors, neutron sources, and medical imaging underscore its significance in modern society. Its unique properties enable advancements in safety, scientific research, and medical diagnostics, contributing to a wide range of beneficial outcomes.
Importance
The importance of americium as a long-lived radioactive element, easy to produce, and versatile lies in its wide-ranging applications and contributions to various fields. These properties make americium an essential component in numerous technologies and scientific advancements.
Americium's longevity as a radioactive element ensures a consistent and reliable source of radiation for applications such as smoke detectors and neutron sources. Its ease of production allows for a steady supply of the element, meeting the demands of these applications. Furthermore, americium's versatility stems from its ability to emit different types of radiation, making it suitable for diverse uses in scientific research, medical imaging, and industrial settings.
The combination of these properties makes americium a crucial element in various industries. Smoke detectors utilizing americium's radioactivity enhance public safety by providing early warnings of fires. Neutron sources employing americium facilitate groundbreaking research in fields such as physics, chemistry, and materials science. Medical imaging techniques incorporating americium aid in accurate diagnoses and monitoring of medical conditions.
In summary, the importance of americium as a long-lived radioactive element, easy to produce, and versatile stems from its unique properties that enable advancements in safety, scientific research, and medical diagnostics.
Benefits
The element named after a continent, americium, plays a vital role in enhancing smoke detection, cancer treatment, and medical imaging advancements, offering tangible benefits that impact our daily lives and contribute to scientific progress.
Americium's unique properties, particularly its long-lived radioactivity and versatility, make it an ideal component in smoke detectors. The ionization process in smoke detectors, enabled by americium's radiation, provides early and reliable detection of smoke particles, significantly reducing the risk of fire-related accidents and saving lives.
In the medical field, americium contributes to advancements in cancer treatment. Neutron sources utilizing americium-241 emit neutrons that can be precisely targeted to destroy cancerous cells, offering an effective and less invasive treatment option. Additionally, americium's applications in medical imaging, such as bone mineral densitometry, aid in the accurate diagnosis and monitoring of bone-related conditions, empowering healthcare professionals to make informed decisions.
The connection between americium and these benefits highlights the importance of understanding the element's properties and harnessing them for practical applications. Americium's unique characteristics make it an essential component in technologies that enhance public safety, improve healthcare outcomes, and contribute to scientific research. Recognizing the significance of americium in these areas fosters a deeper appreciation for the element and its role in shaping our world.
Historical Context
The connection between the historical context of americium's discovery and its name, which is derived from the Americas, offers a unique insight into the intersection of scientific discovery and geographical exploration.
The naming of americium pays homage to the discovery of the Americas, recognizing the continent where the element was first synthesized. This act of recognition highlights the significance of the Americas as a birthplace of scientific advancements and the global nature of scientific collaboration.
Understanding the historical context of americium's name not only adds to the element's story but also underscores the importance of recognizing the contributions of different regions and cultures to the advancement of science.
Environmental Impact
The connection between "Environmental Impact: Radioactive waste management considerations" and "what element is named after a continent" lies in the element's radioactive nature and its potential impact on the environment. Americium, the element named after the Americas, is a radioactive substance that requires careful handling and disposal. Radioactive waste management is a critical aspect of americium's life cycle, as improper disposal can lead to environmental contamination.
Americium's radioactivity stems from its unstable atomic structure. This instability leads to the emission of ionizing radiation, which can be harmful to living organisms. Radioactive waste management practices aim to minimize the environmental impact of americium by safely storing and disposing of radioactive waste. This involves isolating the waste from the environment and preventing its release into the ecosystem.
Understanding the environmental impact of americium and the importance of radioactive waste management is crucial for ensuring the safe use and disposal of this element. Proper waste management practices help protect human health and the environment from the potential hazards of radiation exposure.
Future Prospects
Americium, the element named after the Americas, holds promising prospects for future applications in the fields of energy and medicine, driven by ongoing research and advancements in technology.
- Energy Production: Potential in Nuclear Reactors
Americium's unique properties make it a potential fuel source for nuclear reactors. Research is underway to develop americium-based fuels that could enhance the efficiency and safety of nuclear power generation.
- Medical Applications: Targeted Cancer Treatment
Americium's radioactive nature can be harnessed for targeted cancer treatment. Researchers are exploring the use of americium-based isotopes in brachytherapy, a technique that involves placing radioactive sources directly into or near tumors to deliver precise radiation doses.
- Medical Imaging: Advancements in Diagnostic Techniques
Americium-241 is already used in medical imaging techniques such as bone mineral densitometry. Ongoing research aims to develop new americium-based isotopes for other imaging applications, potentially improving diagnostic capabilities and disease detection.
- Space Exploration: Powering Deep Space Missions
Americium's long-lived radioactivity makes it a potential power source for deep space missions. Research is focused on developing compact and efficient americium-based radioisotope thermoelectric generators (RTGs) to provide reliable power for spacecraft venturing far from the Sun.
The future prospects of americium in energy and medicine are promising, with ongoing research paving the way for innovative applications. As scientists continue to explore the potential of this element, we can anticipate advancements that will benefit society and shape the future of these fields.
FAQs on "What Element is Named After a Continent"
This section addresses frequently asked questions related to the element named after a continent, providing concise and informative answers to common concerns or misconceptions.
Question 1: What is the name of the element that is named after a continent?
Answer: Americium is the name of the element that is named after the Americas.
Question 2: Why is americium named after the Americas?
Answer: Americium was discovered in 1944 at the University of California, Berkeley, and named after the Americas to honor the continent where it was first synthesized.
Question 3: What are the key properties of americium?
Answer: Americium is a radioactive element with a silvery-white appearance. It is a relatively soft metal and is relatively easy to produce.
Question 4: What are the main applications of americium?
Answer: Americium is used in a variety of applications, including smoke detectors, neutron sources, and medical imaging.
Question 5: What are the potential hazards of americium?
Answer: Americium is a radioactive element and can be harmful if not handled properly. It is important to follow proper safety procedures when working with americium.
Question 6: What is the future outlook for americium?
Answer: Americium is a promising element with a wide range of potential applications. Ongoing research is focused on developing new and innovative uses for americium, particularly in the fields of energy and medicine.
These FAQs provide a concise overview of key information related to the element named after a continent, americium. By addressing common questions, we aim to foster a better understanding of this element and its significance.
Transition to the next article section:
Tips Related to "What Element is Named After a Continent"
Understanding the element named after a continent, americium, involves exploring its unique properties, applications, and implications. Here are several tips to enhance your comprehension of this topic:
Tip 1: Explore Americium's Discovery and HistoryDelve into the historical context of americium's discovery at the University of California, Berkeley, and the rationale behind naming it after the Americas. This provides a deeper appreciation for the element's origin and recognition.Tip 2: Understand Americium's Properties and CharacteristicsFamiliarize yourself with americium's fundamental properties, including its atomic number, symbol, and physical and chemical characteristics. This knowledge lays the groundwork for understanding its behavior and applications.Tip 3: Learn about Americium's ApplicationsExplore the diverse applications of americium in fields such as smoke detection, neutron sources, and medical imaging. Comprehending these applications highlights the element's practical significance and societal impact.Tip 4: Consider Americium's Environmental ImpactRecognize the importance of responsible handling and disposal of americium due to its radioactive nature. Understanding radioactive waste management practices ensures the safe and environmentally conscious use of this element.Tip 5: Explore Americium's Future ProspectsStay informed about ongoing research and advancements related to americium's potential in energy production, medical applications, and space exploration. This knowledge provides insights into the element's future significance and contributions.Summary:By following these tips, you can gain a comprehensive understanding of the element named after a continent, americium. From its discovery and properties to its applications and future prospects, exploring these aspects enhances your knowledge and appreciation for this fascinating element.Conclusion
The exploration of "what element is named after a continent" has unveiled the fascinating world of americium, an element with unique properties and diverse applications. Americium's discovery and subsequent naming after the Americas serve as a testament to the global nature of scientific collaboration and the interconnectedness of our world.
Understanding americium's properties, from its silvery-white appearance to its long-lived radioactivity, is essential for harnessing its potential while ensuring its safe and responsible use. Its applications in smoke detection, neutron sources, and medical imaging highlight its practical significance in enhancing public safety, advancing scientific research, and improving healthcare outcomes.
As we look to the future, americium holds promise for transformative applications in energy production, medical treatments, and space exploration. Ongoing research and innovation in these fields will undoubtedly uncover new and exciting ways to utilize this element for the benefit of society.
In conclusion, the exploration of "what element is named after a continent" not only provides a deeper understanding of americium but also underscores the importance of scientific inquiry, responsible element management, and the pursuit of knowledge for the betterment of our world.
Unveiling The Extraordinary: Leonard Douglas Simmons And His Legacy Of Impact
Stephen Macht: Beyond The Screen, Uncovering His Artistry And Impact
Unveil The Hidden Riches: Unveiling Tim Mickelson's Net Worth

ncG1vNJzZmiooqTDsL%2FTcWWapaNoe6W1xqKrmqSfmLKiutKpmJydo2OwsLmOsJ%2BarF2auaa5xKerZqGjYruiucSdZJqepJq%2Fbq2MnKanrJmjsq%2FAjaGrpqQ%3D